On 09/07/12 4:46 AM, Norsefolk_2@yahoogroups.com wrote:
3a. Re: Pewter casting
Posted by: "Sam F"
Date: Sun Jul 8, 2012 4:19 am ((PDT))
I would say, always go with a modern lead-free pewter alloy. I know we're all serious history buffs, but history is not worth risking your health or well-being over. Lead poisoning is nasty stuff.
I have to strengthen what [earlier poster] Charles has said about being careful with copper based alloys. With the huge increase in base copper costs over the last decade, more and more cheaper metals are being substituted by manufacturers. Here read both Zinc and Lead. I personally had the same low level toxic exposure that Charles warned of this winter. Very nasty. Good news was that I too 'recovered' from the worst of it after a couple of days. I suspect both of us will have built up damage to our tissues (especially potential liver concentrations.) Very, very, double plus un-good.
So - care when purchasing bronze (copper tin) alloys is called for. Always ask for the Materials Data Sheet. In North America, suppliers are required by law to supply this information on request. It may mean dealing in larger quantities and with more expensive industrial suppliers - but toxic heavy metals poisoning is for *life*.
Sam has warned of the same thing with 'pewter'. Modern lead free alloys are typically 92 tin, 6 copper, trace of antimony. The antimony is a problem, but usually is in the range of 1 - 1.5 %. I've said this before, but lead free plumbing solder from your hardware is a safe alloy to use.
One important note however.
Lead in these mixtures does two things. Both are important to our understanding of just how historic casting process will work:
1) Acts to effectively lower the melting point of the metal.
Working with small crucibles in a charcoal fire, you can see why that might influence your results.
2) Acts to reduce the viscosity - improving the flow of the liquid metal. Historically you see lead added to copper alloys for the same root cause as is happening with the degrading of alloys today - cost. Losses during the casting process, and declining access to the correct metals was both accommodated by adding the cheaper and easier to get lead in an effort to 'extend' the valuable copper and tin in the mixes.
There are some ways you can work to reduce the impact of a 'thicker' flow with the modern tin alloys.
1) increase the size of the entry cone 'button' to help force the metal down into the mould
2) pre head your moulds
I have seen modern workers drastically overhead their metal to increase its pouring ability. I most definately would *not* recommend this. All the toxic effects come from metal as vapour - most likely if the metal is overheated. Better just to adjust your mould!
One of the interesting things is taking a look at the existing stone artifact moulds, for hints of what metals might have been used.
 |
| Stone mould for a disk broach |
The reason for this is that the 92 % tin alloy is not only more viscous, it also has a much higher surface tension (which might be related ?). The result is that with a top pour, the metal 'pillows up' , not filling to the edges of the mould. For that reason, all the moulds I have used are designed for edge pour. (This also allows the use of a single backing plate.)
 |
| Past work - Soapstone moulds and cast pieces. |
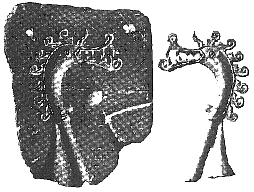 |
| Viking Age slate mould for a small belt mount |
This certainly suggests two things to me:
1) The smith was attempting to be quite careful about just how much metal was being contained in the 'button'.
2) The narrow diameter of the entry line is more suitable for a very 'thin' metal at molten temperatures.
Taken together this suggest a use of bronze or silver as the intended metal for this object.
The melting points of the alloys of these metals ranges about 1800 F, easily four times the temperature required for a lead based pewter. With the mould pre-heated, a successful casing in bronze or silver can be made in a stone mould. (I do wonder if the back side of the artifact mould shows any heating scars.) I have not personally used any slate moulds, but I do know from experience that soapstone suffers some loss of surface detail due to chipping when used with bronze or silver.
Those interested in trying out simple soapstone moulds for pewter casting can find a technique guide I prepared.
Hey D! checking out your blog now that I have an office up and running. Starting this sunday I will be running a short series on pewter over at my blog http://uncannygallery.blogspot.com/ if anyone is interested you can send them over, if not.... that's fine too!
ReplyDeletecheers!